Smart SAP® EWM functions and solutions for the pharmaceutical supply chain
For companies active in the pharmaceutical supply chain, complying with the regulations on the serialization of medicines is a tremendous challenge. For instance, pharmaceutical companies are required to organize the many different types of serial numbers arising out of country-specific regulations, and process responses from various stakeholders in different formats, such as E-Mail, PDF and Excel, to name but a few. These requirements can only be met using a sophisticated and integrated warehouse management system (WMS). We spoke with the Managing Director of KNAPP IT Solutions, Thomas Furthmayr, who sheds some light on the useful functions, benefits and smart solutions that the WMS software SAP® Extended Warehouse Management (SAP® EWM) provides for the pharmaceutical sector.
Before we dive in, could you tell us what SAP® EWM is?
Thomas Furthmayr: SAP® Extended Warehouse Management, SAP® EWM for short, is a logistics software solution in the SAP Supply Chain Management suite. The solution is a comprehensive warehouse management system (WMS) with an integrated warehouse control system (WCS) and material flow system (MFS). SAP® EWM has a range of functions not only for stock management, but also for the flexible and dynamic handling of the material flow in a warehouse.
KNAPP IT Solutions is the SAP® EWM expert in the KNAPP group and we’ve been an official SAP partner since 2012. We offer various implementations, solutions and services on an SAP® EWM-basis with SAP® EWM by KNAPP for a range of different sectors.

What benefits does SAP® EWM by KNAPP offer as a WMS for companies in the pharmaceutical business?
Thomas Furthmayr: SAP® EWM is the ideal warehouse management system for those who rely on an SAP® IT strategy based on SAP® ERP or S/4HANA. As a best-of-suite solution, SAP® EWM can centrally map all business processes and associated logic in a unified way. The harmonized end-to-end SAP® landscape benefits the warehouse management system in a number of ways.
Thanks to the direct coupling of SAP® EWM to the PLC, the high degree of automation usually found in the pharmaceutical sector can be mapped more clearly and reliably: Fewer interfaces and central, comprehensive process control in SAP® EWM reduce the likelihood of errors during data transfer, while increasing communication speed. As an end-to-end solution, SAP® EWM efficiently and easily analyzes errors and their causes.
When we implement an SAP® EWM project for our customers in the pharmaceutical sector, they get a solution that is loaded with best practices coming from our extensive experience in the industry. Our expertise stems from more than 700 projects implemented across the globe, including projects for large companies such as Alliance Healthcare, McKesson, ABCD, Phoenix, Noweda or Sanacorp as well as local healthcare specialists.
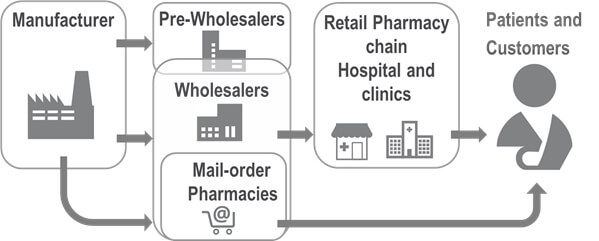
Serialization is a taxing topic in the pharmaceutical supply chain. How can SAP® EWM help?
Thomas Furthmayr: Since February 2019, strict regulations are in place in the EU to ensure a legally conform supply chain in the pharmaceutical sector. Similar regulations have also been made outside of the EU aimed at preventing falsified and counterfeit medicines. However, the definitions of how the supply chain ought to comply with the regulations sometimes differ.
These requirements on process security inadvertently affect the processes in the warehouse and of the warehouse management systems. The SAP® EWM standard version has a serial number management function, which allows all items to be tracked and traced seamlessly along the entire supply chain – from the manufacturer to the different recipients such as pharmacies and hospitals. Even though this version provides a good basis to ensure the end-to-end traceability of serial numbers, the requirements on serialization go beyond process flexibility. Usually, the minimum requirement is that manufacturers publish the serial numbers in a national database and define the market they are allowed to supply. Before a medicine is given to patients, its authenticity is checked by verifying its serial number in the database and deactivating the assigned serial number. The process of publishing and later deactivating serial numbers prevents that patients receive falsified medicines, which have no serial number, an invalid serial number, or two serial numbers.
Some countries do not require the seamless tracking of serial numbers along the entire supply chain. In the EU, for instance, medicines do not normally have to be tracked on their way from the manufacturer to the wholesaler or from the wholesaler to the pharmacy. However, if the medicines are exchanged between different wholesalers, their authenticity must be checked. If the medicines are directly delivered to patients according to the direct-to-patient principle, for instance, or if the customer, like hospitals, does not have the means to deactivate the serial numbers, wholesalers are additionally required to deactivate the products and map this in the warehouse management system.

Can the different regulations on serialization be met using SAP® EWM by KNAPP?
Thomas Furthmayr: Yes. By building on the SAP® EWM standard, we have integrated solutions into SAP® EWM by KNAPP to meet these requirements. By extending the master data, the pharmaceutical products are given the attribute “obligatory pharmaceutical serialization” and the process steps required for each country are saved. All this data can be maintained and updated by the customer themselves should the requirements change. In addition, a layer of interfaces makes communication with the country-specific databases possible using a web service and maps both the process integration of all message types and the required measures for error handling.
What do the serialization processes look like in SAP® EWM by KNAPP with regard to goods-in and goods-out, for example?
Thomas Furthmayr: For goods-in, SAP® EWM by KNAPP includes the messaging and querying functions required by the different regulations on serialization. One of the required functions is the risk-based verification of unqualified partners, a different wholesaler, for instance. For deliveries of mail-order medicines to end customers or for medicine distribution to delivery points without check-out possibilities, the solution also allows the numbers to be logged during the picking process or at special stations. Customers using our Vision Central Belt benefit from the system’s special camera technology, which automatically records serial numbers during picking. The serial numbers are booked by SAP® EWM by KNAPP, eliminating the need for further time-consuming manual steps.

Batch management is another integral part of the pharmaceutical supply chain. How does SAP® EWM by KNAPP come into play here?
Thomas Furthmayr: SAP® EWM includes a wide range of functions for batches and managing batches, from goods-in to shipping. With our solution, incoming batches can be directly logged during delivery and different features saved in the system, such as supplier and date marks. SAP® EWM also helps users meet other requirements in the pharmaceutical business. For example, they can pick according to remaining shelf life for selected customers and allocate specific batches. All batches are visible on the warehouse management monitor and can be selected according to stock and features at all times.
SAP® EWM also allows batches to be locked, which is a useful function for recalling single or multiple batches from a supplier. In this case, users should make sure that picking is halted for the selected batches and that any goods picked from this batch are not shipped but replaced. Batches not known to SAP® EWM are saved in a “blacklist”, ensuring that they are identified and immediately locked should they resurface in future. Finally, all these processes can be reliably mapped in SAP® EWM by KNAPP.
What other useful functions does SAP® EWM by KNAPP provide for the pharmaceutical sector?
Thomas Furthmayr: In its standard version, SAP® EWM covers all the important functions of a professional warehouse management system. Along with requirements around serialization and batch management, SAP® EWM by KNAPP helps our customers from pharmaceutical wholesale and pre-wholesale meet even more sector-specific requirements, offering:
In which ways can SAP® EWM by KNAPP be introduced as a warehouse management system?
Thomas Furthmayr: Thanks to the adaptability and scalability of the warehouse management system, SAP® EWM is suitable for any level of automation, regardless of whether the warehouse is RF-/voice-guided or fully automatic. Our service portfolio includes a range of ways to implement as well as optimize existing SAP® EWM solutions in the pharmaceutical sector.
For example, our Healthcare Model Company for SAP® EWM helps eliminate lengthy specifications phases. It includes all the important core processes from goods-in to product-friendly storage and quality checks to goods-out – all configured to suit the requirements in the pharmaceutical sector. Automation solutions typically used in the sector, such as central belt picking systems or goods-to-person picking systems, are also displayed in the Healthcare Model Company and integrated in SAP® EWM.
A professional emulation software is used in a workshop setting to show how the different warehouse technologies and business cases of the customer would run using SAP® EWM. Any additional procedures or requirements that the customer might have are defined together with them and implemented as quickly as possible. This reduces time, costs and risk in the introduction of SAP® EWM.
Besides greenfield projects, we have specialized in projects using SAP® EWM to retrofit existing, non-SAP, warehouse management and warehouse control systems. Customers benefit from our wealth of experience integrating automation technology from KNAPP and many other manufacturers into SAP® EWM through retrofitting projects. If a customer uses different warehouse management systems, we help them create the ideal template design in SAP® EWM according to the specifications of the warehouse and sector and help with the international rollout.
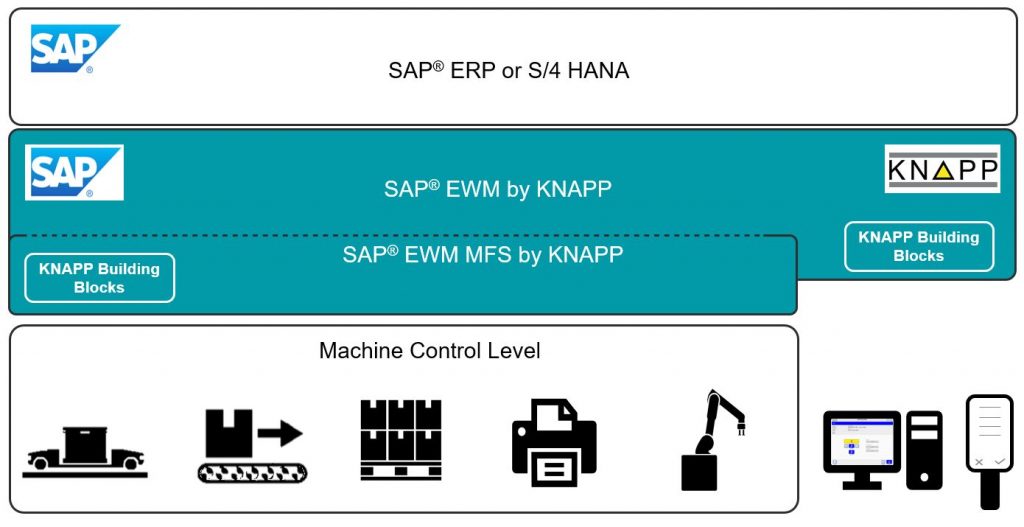
What can you offer customers who already use SAP® EWM?
Thomas Furthmayr: We offer support using SAP® EWM spot consulting, optimizing both completed and current EWM implementation processes. In addition, our ITIL®-certified 24/7 SAP® EWM Service Desk provides support to those SAP® EWM users who have introduced the solution themselves or through a different SAP partner.
We implement our projects using our extensive process expertise and KNAPP Building Blocks. KNAPP Building Blocks are SAP® EWM software and configuration modules that extend the standard version of SAP® EWM with solutions for the sector-specific requirements of the pharmaceutical sector. SAP® EWM helps to map processes, extend functions and seamlessly integrate automation solutions used in the sector.
Further reading recommendations

What are 11 questions should you absolutely ask yourself before carrying out an SAP® EWM implementation project? Read on and find out in this blog post.



